FERROFLUIDS : biocompatible oily ferrofluid for better thermal tumor ablation efficiency
Domain Health and wellbeing
Technologies
Nanotechnology
Personalized medicine
Challenges
Aqueous ferrofluids used in magneto-induced hyperthermia treatment require high concentrations of iron oxide nanoparticles. Indeed, water is a too efficient coolant and does not allow controlling the thermal dose deposited by the ferrofluid at target site. In addition, the injected hydrophilic nanoparticles can diffuse, dilute or aggregate in the various tumor compartments (interstices of epithelia, ECM, etc.), which reduces their thermal power and leads to loss of control of the thermal dose to be effectively delivered for treatment. By improving heat transfers toward the tumor, dispersion of superparamagnetic NPs in a biocompatible lipid phase permits to increase 10 times the thermal power transferred compared with dispersion in an aqueous medium. This gain, associated with the confinement of the NPs in an oily phase, opens the way to more precise treatment by allowing injections of microvolumes and better spatiotemporal control of the thermal dose while using lower iron oxide dose.
Innovative solution
This technology is based on the colloidal stabilization of magnetic nanoparticles confined in an oily heat transfer medium, provided by fine control of phospholipid grafting onto the magnetic nanoparticle surface. Under magnetic induction, this stabilization prevents the formation of magnetic dipole interactions, which can drastically reduce the dissipated heating power of magnetic nanoparticles.
Applications
Cancerology :
-
Tumor and lymph node thermo-ablation
-
Thermo-sensitization of tumor microenvironment in immunotherapy
-
Potentiation of chemotherapies
-
Image-guided therapy (MRI, Optical…)
Competitives advantages
-
Efficient heat transfer medium for better control of the thermal dose deposited locally on cancer tissues
-
Usable as carrier fluid of lipophilic drugs
-
Imaging-guided therapy tool opening access to multimodality (MRI, Optical imaging,...)
-
Can be emulsified for parenteral or intravenous administration
-
Biocompatibility
-
Versatile process and stable products (up to several months)
DEVELOPMENT STATUS:
- Preclinical developments
- Calibration of the thermal doses to be delivered at target sites to reach biological effects (sensitization and ablation) according to the thermal heating power of the magnetic NPs, the sample volume and the clinical AMF conditions applied.
- Evaluation of the long-term toxicity effect of the product
How it works
The innovation relates to a composition called "lipid Ferrofluid" consisting of iron oxide maghemite (ϒ-Fe2O3) nanoparticles dispersed in a carrier oily fluid, characterized in that:
- the nanoparticles are in the form of a phospholipid/iron oxide complex
- the nanoparticles are dispersed in the fluid
- the fluid is a lipid oil based on a mixture of fatty acid esters.
In particular, the fluid contains triglycerides or propylene glycol esters of medium-chain saturated fatty acids (e.g. Miglyol 840® or 812N®). Preferably, the phospholipid is DOPA (1,2-Dioleoyl-sn-glycero-3-phosphatidic acid) or DOPS (1,2-Dioleoyl-SN-glycero-3-phospho-L-serine).
The colloidal stabilization of magnetic nanoparticles without surfactants is obtained in biocompatible (oily) lipid media composed of fatty acid esters thanks to the fine control of phospholipid surface grafting. Indeed, the grafting rate of phospholipids must be optimized to promote solvation by the molecules constituting the oil (Fig. 1). Colloidal stability is of utmost importance under an alternating magnetic field (AMF) for keeping heating performance in magnetic hyperthermia since the magnetic dipole interactions which lead to magnetic aggregates are at the origin of the reduction in heating efficiency of nanoparticles under AMF induction.
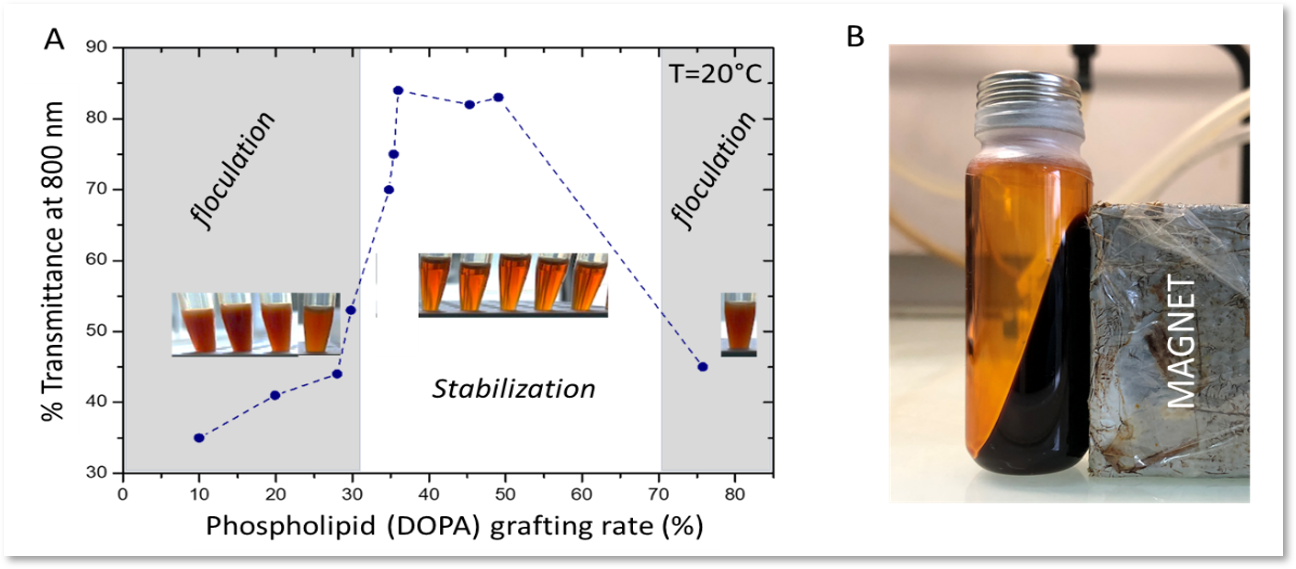 Figure 1. Dispersion properties of magnetic nanoparticles in oil as a function of DOPA grafting density (A) and picture of a magnetic oily fluid obtained according the process (B).
Figure 1. Dispersion properties of magnetic nanoparticles in oil as a function of DOPA grafting density (A) and picture of a magnetic oily fluid obtained according the process (B).
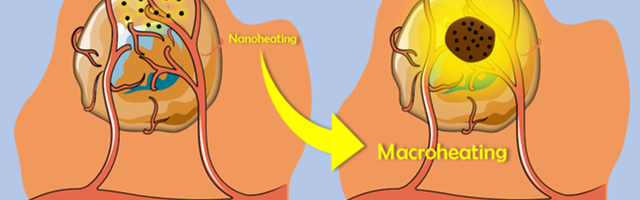
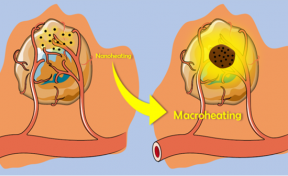
The confinement of the magnetic nanoparticles makes it possible to store in the oily phase the heat that is dissipated at the nanoscopic scale by the magnetic nanoparticles, which will then be returned macroscopically into the tumor (Fig. 2). This strategy, using an intermediate phase ensuring the heat transfer, permits to limit unavoidable thermal losses and to take full advantage of the intrinsic high heating power of optimized iron oxide nanoparticles under magnetic induction.
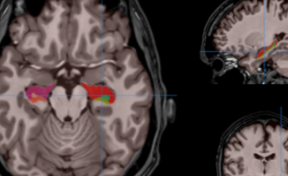
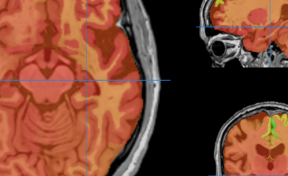
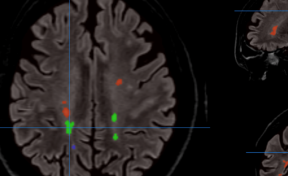
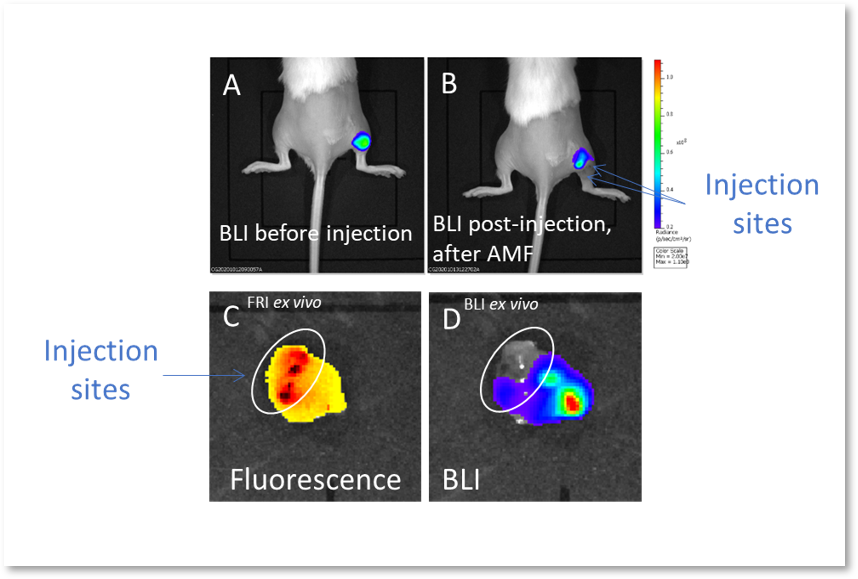 Figure 2. (A) Bioluminescence image (BLI) attesting of the viability of tumor cancer cells before injection and AMF application; (B) BLI showing the partial tumor ablation and the two injection sites on the subcutaneous tumor of the oily FF (2 1 µL, CFe2O3 = 300 mg/mL) after AMF application (15 min at 473.5 kHz, 13.4 kA·m-1). (C) fluorescence and (D) bioluminescence imaging of the excised tumor showing the co-localisation of the fluorescent oily ferrofluid at 800 nm by addition of indocyanin green (ICG) with the thermo-ablated zones generated by the hotspots.
Figure 2. (A) Bioluminescence image (BLI) attesting of the viability of tumor cancer cells before injection and AMF application; (B) BLI showing the partial tumor ablation and the two injection sites on the subcutaneous tumor of the oily FF (2 1 µL, CFe2O3 = 300 mg/mL) after AMF application (15 min at 473.5 kHz, 13.4 kA·m-1). (C) fluorescence and (D) bioluminescence imaging of the excised tumor showing the co-localisation of the fluorescent oily ferrofluid at 800 nm by addition of indocyanin green (ICG) with the thermo-ablated zones generated by the hotspots.
The gain obtained in terms of thermal power with oily ferrofluids (Fig. 3) increases with the intrinsic heating power of the nanoparticles involved, which makes it possible to adapt the thermal doses to be applied according to the type of treatment (sensitization, potentiation, ablation) and allows reducing the amount of iron oxide to inject. The heat transfer performance is maintained even for microvolumes (of the order of 1 µL) which is not possible with aqueous ferrofluids (Fig. 3, 4). This property can be used to generate hotspots to treat extremely small tumor nodules.
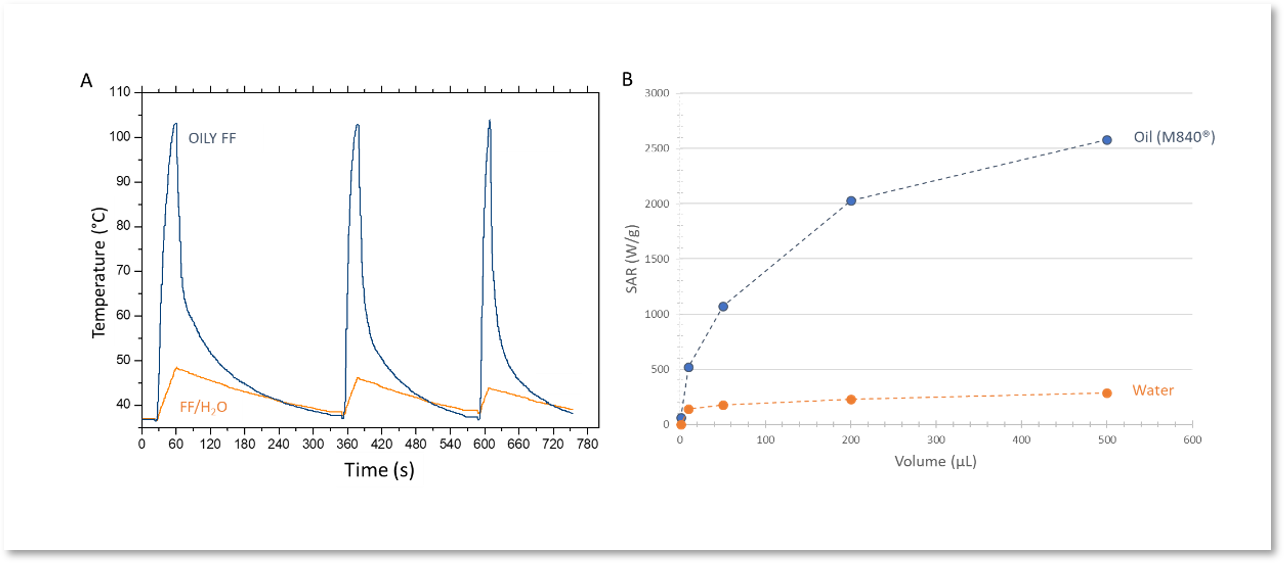 Figure 3. (A) Kinetic temperature profiles of 1 mL of oily ferrofluid (CFe2O3 = 5 g/L, AMF: 473.5 kHz, 13.4 kA·m-1, regulating temperature T0 = 37°C) compared with aqueous ferrofluid in the same conditions. (B) Effect of volume on thermal power (SAR, specific absorption rate) values of oily ferrofluid compared to aqueous ferrofluid (CFe2O3 = 5 g/L, AMF: 473.5 kHz, 13.4 kA·m-1, T0 = 37°C).
Figure 3. (A) Kinetic temperature profiles of 1 mL of oily ferrofluid (CFe2O3 = 5 g/L, AMF: 473.5 kHz, 13.4 kA·m-1, regulating temperature T0 = 37°C) compared with aqueous ferrofluid in the same conditions. (B) Effect of volume on thermal power (SAR, specific absorption rate) values of oily ferrofluid compared to aqueous ferrofluid (CFe2O3 = 5 g/L, AMF: 473.5 kHz, 13.4 kA·m-1, T0 = 37°C).
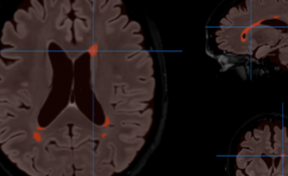
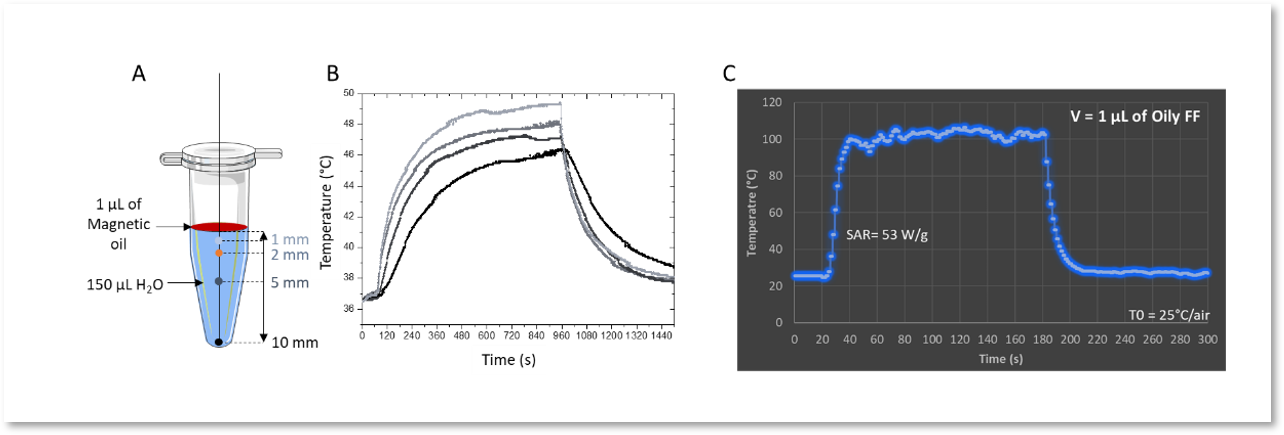 Figure 4. A and B: Kinetic temperature profiles of a volume of 150 µL of water heated by 1 µL of oily ferrofluid (CFe2O3 = 300 g/L, AMF : 473.5 kHz, 13.4 kA·m-1, 15 min, T0 = 37°C); (C) Kinetic temperature profile of 1 µL of oily ferrofluid at 473.5 kHz, 13.4 kA·m-1 (CFe2O3 = 300 g/L).
Figure 4. A and B: Kinetic temperature profiles of a volume of 150 µL of water heated by 1 µL of oily ferrofluid (CFe2O3 = 300 g/L, AMF : 473.5 kHz, 13.4 kA·m-1, 15 min, T0 = 37°C); (C) Kinetic temperature profile of 1 µL of oily ferrofluid at 473.5 kHz, 13.4 kA·m-1 (CFe2O3 = 300 g/L).
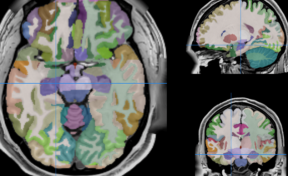
Advantageously, the ferrofluid is biocompatible, administrable by intra-tumoral injection (e.g. by convective enhanced diffusion in brain or chemoembolization in liver…) or by intravenous way in its nano-emulsion form. Lipophilic drugs as well as fluorescent dyes can be dissolved in oily ferrofluids (Fig. 2C) as part of strategies combining chemotherapy and thermotherapy monitored by multimodal (MRI / optical) imaging tools.
Inventors
Developed by a research team : ICMCB-LCPO-ARNA-IMOTION
IP
1 patent : FR1913249 - FR3103376A1 (5/28/21) - PCT/FR2020/052184
PARTERSHIPS
License and/or R&D collaboration
Contact
Carlos LARRAYA
%63%2e%6c%61%72%72%61%79%61%40%61%73%74%2d%69%6e%6e%6f%76%61%74%69%6f%6e%73%2e%63%6f%6d
+33 (0)5 33 51 43 11